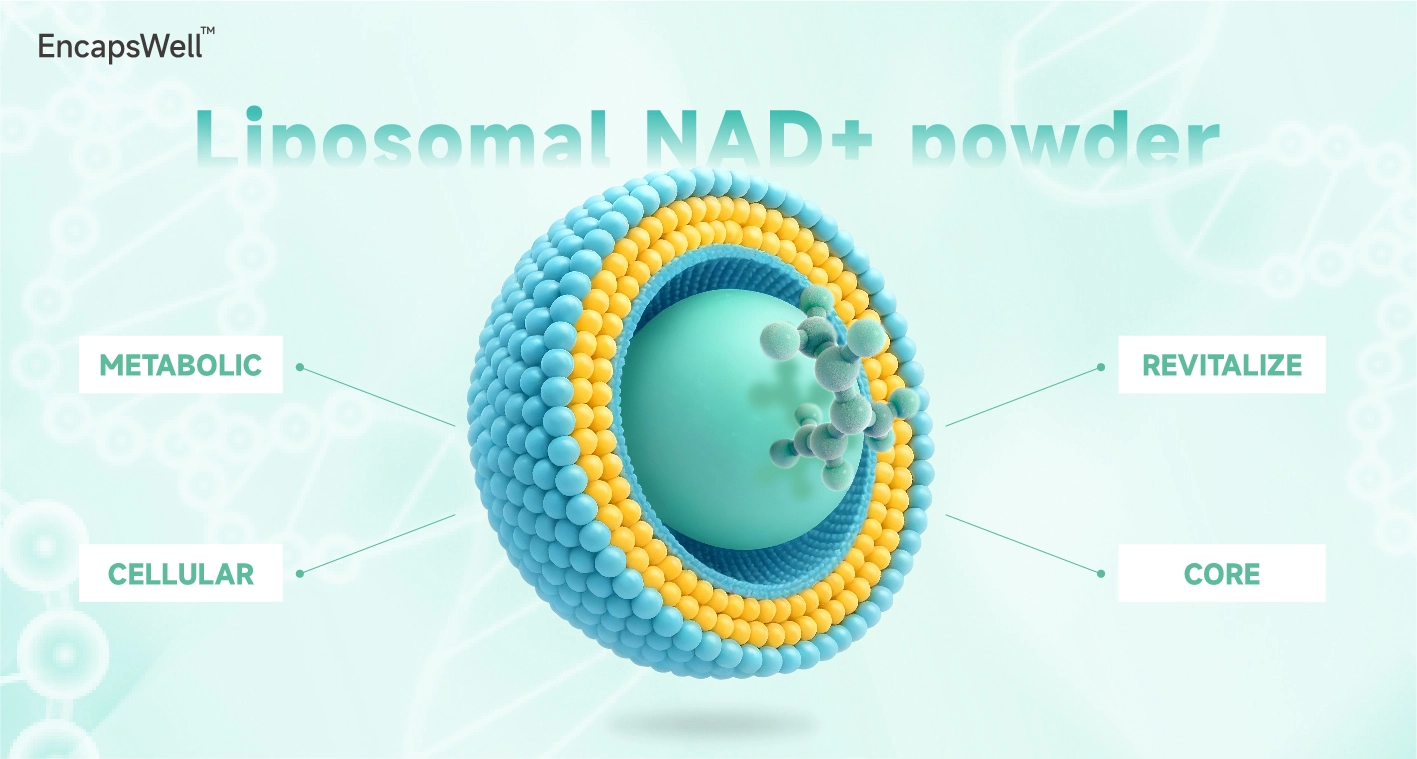
Liposomal NAD+ Powder
NAD+ content ≥ 98%
Liposomal encapsulation increases absorption by up to 5–10× vs standard powder
Water-soluble powder, suitable for beverage or supplement formulation
Certifications & Quality:
ISO9001 / HALAL / NOP / EOS / KOSHER / cGMP / ISO22000 / FSSC22000 / HACCP / Non-GMO / Vegan
Third-party tested for purity, potency, and heavy metals
Procurement Highlights:
OEM/ODM customization available
Flexible dosage forms: Powder, Tablets, Hard Capsules, Soft Capsules, Liquid-ready formulations
EncapsWell™ Liposomal NAD+ Powder - Advanced Cellular Energy Support
EncapsWell™ Liposomal NAD+ Powder leverages advanced phospholipid encapsulation to overcome the inherently low bioavailability of standard NAD+ supplements. By shielding the active molecules from gastric degradation, our formulation significantly enhances cellular uptake and mitochondrial integration. Backed by HPLC-verified purity (≥98%), this premium ingredient is engineered for brands seeking pharmaceutical-grade stability in their cellular health product lines.

What Makes Our Liposomal NAD+ Powder Special?
Advanced Delivery System
We utilize a proprietary sonication and high-pressure homogenization process to create uniform, nano-sized liposomes. These lipid bilayers mimic human cell membranes, facilitating direct fusion and bypassing the traditional intestinal transporters that often bottleneck NAD+ absorption.
Pure and Potent Formula
Every group contains NAD+ with ≥98% virtue, confirmed through thorough HPLC testing. We utilize as it were pharmaceutical-grade fixings with total traceability documentation, guaranteeing you get precisely what's on the label.
Versatile Powder Format
Mix effortlessly into water, smoothies, or your favorite refreshment. This water-soluble powder gives you total control over dosing and timing, making it idealize for your every day routine.
Key Benefits for Your Health
Cellular Energy Enhancement
NAD+ serves as a pivotal coenzyme in cellular vitality generation. By supporting mitochondrial work, our supplement makes a difference combat weariness and advances maintained vitality all through your day.
Cognitive Performance Support
Your brain requires colossal sums of vitality to work ideally. Our liposomal NAD+ powder crosses the blood-brain boundary proficiently, conveying fuel specifically to brain cells for improved center and mental clarity.
Recovery and Repair
NAD+ enacts sirtuins, proteins known as "life span qualities" that back DNA repair and cellular upkeep. This handle gets to be particularly critical as we age and our normal NAD+ levels decline.
Metabolic Health
Support sound digestion system by improving your cells' capacity to change over supplements into usable vitality. This progressed productivity can advantage everything from work out execution to every day vitality.
Who Benefits Most From Liposomal NAD+ Powder?
High-Performance Professionals
If you confront requesting mental challenges, long work hours, or visit travel, NAD+ supplementation can offer assistance keep up cognitive sharpness and diminish recuperation time from stress.
Fitness Enthusiasts
Athletes and dynamic people utilize our powder to improve work out execution, diminish muscle soreness, and quicken recuperation between preparing sessions.
Health-Conscious Adults
Those centered on solid maturing appreciate NAD+'s part in cellular upkeep and its potential to bolster life span pathways actually actuated by our bodies.
B2B Supply & Customization Services
As a vertically integrated manufacturer, EmerWell Bio supports global distributors and formula developers with:
-
Bulk Supply: Scalable production capacity to meet high-volume demands.
-
OEM/ODM Support: Custom particle sizing and lipid ratios tailored to your specific delivery requirements (Liquid vs. Powder).
-
Full Documentation: Each batch is accompanied by a Certificate of Analysis (COA), MSDS, and Stability Data.
-
MOQ & Sampling: Flexible trial orders available for R&D evaluation and pilot production.
Scientific Foundation
The Stability Challenge in NAD+ Formulation While Nicotinamide Adenine Dinucleotide (NAD+) is the essential coenzyme for SIRT1 activation and DNA repair, its molecular instability makes it prone to rapid oxidation. EncapsWell™ solves this by creating a protective environment, ensuring that the NAD+ remains potent throughout its shelf life and delivers consistent physiological impact in every dose.
Research shows that maintaining optimal NAD+ levels supports:
· Mitochondrial efficiency
· Cellular repair mechanisms
· Healthy inflammatory response
· Metabolic flexibility
· Cognitive function
Our liposomal delivery system addresses the primary challenge with NAD+ supplementation: bioavailability. Traditional NAD+ supplements often break down before reaching your cells, limiting their effectiveness.
Quality You Can Trust
Comprehensive Certifications
Our manufacturing facility maintains multiple quality certifications including ISO9001, cGMP, ISO22000, FSSC22000, and HACCP. These credentials ensure every batch meets the highest safety and purity standards.
Stringent Quality Control & Traceability
Beyond our internal ISO-certified labs, every batch undergoes third-party verification via HPLC and LC-MS/MS to ensure zero contamination (heavy metals, residual solvents, or microbial impurities). We provide full batch transparency, allowing our partners to maintain the highest compliance standards for their end markets.
Clean Label Promise
We believe in transparency. Our formula contains no artificial additives, preservatives, or unnecessary fillers. It's vegan-friendly, non-GMO, and suitable for various dietary preferences.
|
ITEMS |
STADNARD |
RESULTS |
METHOD |
|
Assay(NAD) |
50% |
50.7% |
HPLC |
|
Physical Control |
|||
|
Appearance |
Light yellow to white powder |
Conforms |
Visual |
|
Loss on Drying |
≤8% |
2.33% |
Eur.Ph.7.0 [2.8.17] |
|
Ash |
≤5% |
1.98% |
Eur.Ph. <2.4.16> |
|
Chemical Control |
|||
|
Total Heavy Metals |
≤10 ppm |
Conforms |
ICP-MS or equivalent or GB |
|
As |
≤1ppm |
0. 10ppm |
ICP-MS or equivalent or GB |
|
Pb |
≤2ppm |
0. 10ppm |
ICP-MS or equivalent or GB |
|
Cd |
≤1ppm |
0. 04ppm |
ICP-MS or equivalent or GB |
|
Hg |
≤0. 1ppm |
0.005ppm |
ICP-MS or equivalent or GB |
|
Microorganism Control |
|||
|
Total Plate Count |
≤10000 cfu/g |
Conforms |
AOAC or equivalent or GB |
|
Yeast and Mold |
≤100 cfu/g |
Conforms |
AOAC or equivalent or GB |
|
Coliforms |
Negative |
Negative |
AOAC or equivalent or GB |
|
E.Coli |
Negative |
Negative |
AOAC or equivalent or GB |
|
Staphylococcus |
Negative |
Negative |
AOAC or equivalent or GB |
|
Salmonella |
Negative |
Negative |
AOAC or equivalent or GB |
|
Conclusion |
|||
|
Standard |
Conform with specification. |
||
|
Storage |
Store in cool and dry places. Keep away from strong light and heat. |
||
|
Packing |
Pack in 25kgs paper-drums, inner by double plastic bag |
||
|
Shelf life |
24 months under the above condition,and in it’s original package |
||

How to Use
Start with one serving (as recommended on packaging) mixed into 6-8 ounces of cold water or your preferred beverage. Take on an empty stomach for optimal absorption, preferably in the morning to support all-day energy.
For best results, use consistently as part of a healthy lifestyle that includes balanced nutrition, regular exercise, and adequate sleep.
The EmerWell Bio Difference
At EmerWell Bio, we combine cutting-edge science with rigorous quality standards. Our team of PhD scientists brings decades of pharmaceutical and biotech experience to nutritional supplement development.
Backed by our parent company Wellgreen's 12+ years of manufacturing expertise, we control every step of production from ingredient sourcing to final packaging. This vertical integration ensures consistent quality and enables us to offer competitive pricing without compromising standards.
Choose Advanced Nutrition
Your cells deserve the best fuel available. Our product represents the next generation of cellular nutrition - scientifically formulated, rigorously tested, and designed for real results.
Experience the difference that superior absorption and pharmaceutical-grade purity can make in your energy, focus, and overall vitality.
Contact us
Ready to optimize your cellular health? Contact us at info@emerwell-bio.com to learn more about our premium liposomal NAD+ powder supplements and discover how we can support your wellness journey.
Have a project in mind? Tell us your goals — we’ll help you make it real.