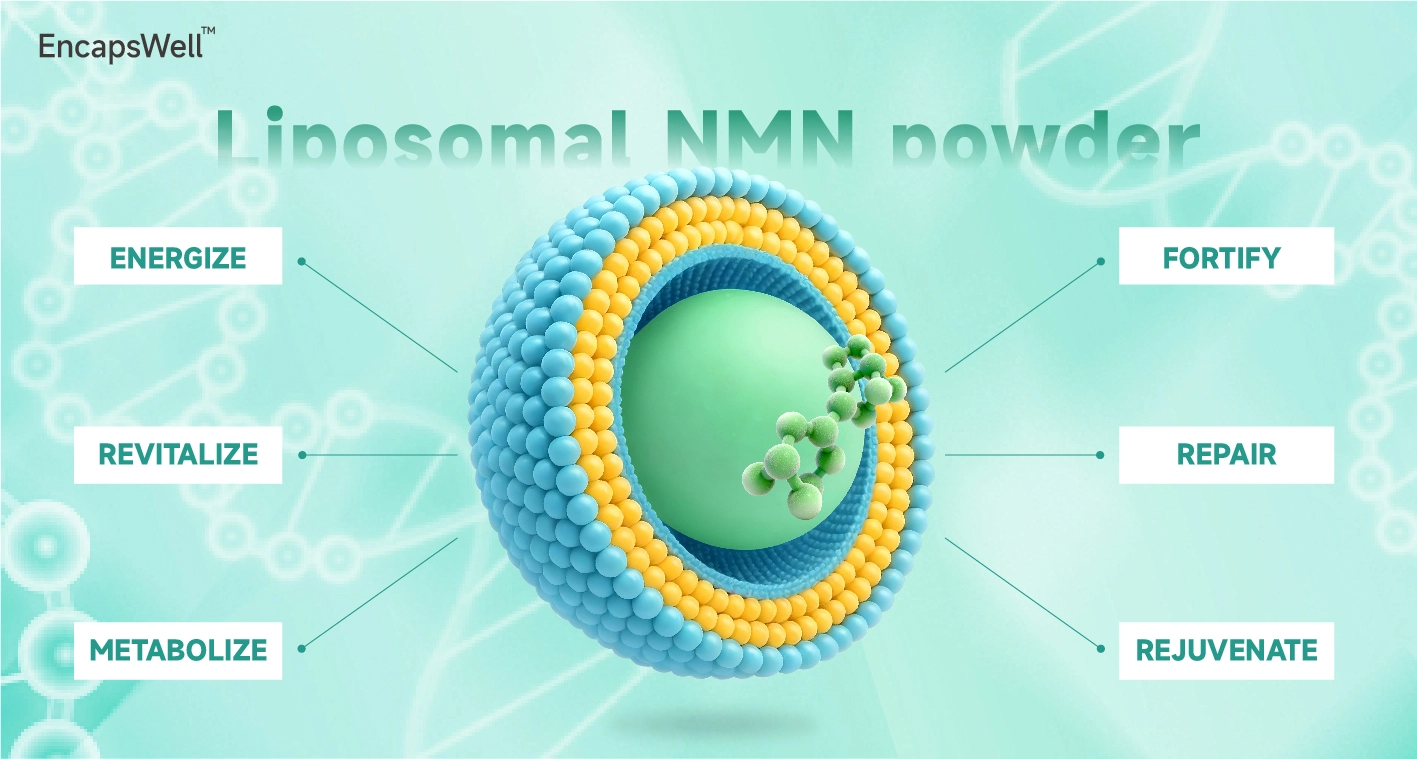
Liposomal NMN Powder
NMN content ≥ 99%
Liposomal delivery improves absorption 4–8× vs unencapsulated powder
Shelf-stable powder for easy formulation
Certifications & Quality:
ISO9001 / HALAL / NOP / EOS / KOSHER / cGMP / ISO22000 / FSSC22000 / HACCP / Non-GMO / Vegan
Third-party verified for quality and potency
Procurement Highlights:
OEM/ODM friendly
Flexible dosage forms: Powder, Tablets, Hard Capsules, Soft Capsules, Liquid-ready formulations
EncapsWell™ Liposomal NMN Powder: Advanced Cellular Energy Support
Are you looking for an effective way to boost your cellular vitality and support healthy aging? Our EncapsWell™ liposomal NMN powder offers cutting-edge liposomal delivery technology that upgrades assimilation by 4-8 times compared to standard NMN supplements. This inventive detailing conveys exceedingly bioavailable Nicotinamide Mononucleotide specifically to your cells, where it underpins NAD+ generation for ideal cellular function. With 99% purity and third-party confirmed quality, you can believe ourproduct to convey results.
| Purity: | ≥99% (HPLC Verified) |
| Stability: | High thermal stability due to liposomal shielding. |
| Bulk Density: | Optimized for precise capsule filling. |
| pH Stability: | Resistant to low gastric pH environments. |
What Makes Our Liposomal NMN Powder Formulation Special?
Our progressed liposomal innovation changes how your body absorbs and metabolizes NMN. The nanoscale liposomes make a protective lipid bilayer around each NMN atom. This inventive conveyance framework guarantees the dynamic fixings reach your cells intaglio and prepared to work.
Unlike conventional supplements that lose strength amid absorption, our definition keeps up soundness all through your stomach related framework. The phospholipid bilayer structure mirrors your characteristic cell layers. This compatibility permits for consistent cellular take-up and greatest bioavailability.
Key Benefits for Your Health
Enhanced Cellular Energy Production
Your mitochondria are your cellular powerhouses. Our product supports these cellular powerhouses by boosting NAD+ levels normally. This handle makes a difference combat weariness and keeps up unfaltering vitality all through your day.
DNA Repair and Longevity Support
NAD+ plays a pivotal part in actuating sirtuins, regularly called "life span proteins." These proteins bolster DNA repair components and offer assistance keep up genome solidness. This cellular upkeep is fundamental for sound aging.
Cognitive Function Enhancement
Your brain requests noteworthy vitality to work ideally. By supporting mitochondrial wellbeing in brain cells, our definition makes a difference keep up sharp considering and clear decision-making abilities.
Metabolic Health Optimization
Healthy digestion system depends on effective cellular vitality generation. Our supplement bolsters liver work and makes a difference optimize how your body forms fats and carbohydrates.
Perfect for Your Lifestyle Needs
High-Performing Professionals
EncapsWell™ Liposomal NMN is engineered for superior metabolic stability. Standard NMN is often degraded into Nicotinamide in the digestive tract before it can reach systemic circulation. Our phospholipid bilayer protection minimizes this premature conversion, significantly boosting NAD+ precursor availability for DNA repair and mitochondrial sirtuin activation.
Fitness Enthusiasts and Athletes
Whether you're preparing for competition or keeping up wellness, our liposomal NMN powder supports ATP amalgamation for way better continuance. It moreover quickens muscle cell repair and decreases recuperation time between workouts.
Health-Conscious Individuals
If you're proactive almost aging gracefully, keeping up NAD+ levels is a savvy procedure. Our supplement underpins cardiovascular wellbeing, cognitive work, and metabolic wellness as you age.
Nutricosmetic Formulators
Cellular wellbeing specifically impacts how you see and feel. By advancing cell digestion system and repair, our detailing makes a difference move forward skin brilliance, versatility, and generally vitality.
Quality You Can Trust
We manufacture under strict cGMP standards with comprehensive quality testing. Every batch undergoes third-party verification for purity and potency.

Why Choose EmerWell Bio?
Our group incorporates PhD-level researchers with pharmaceutical and biotech foundations. We do not take after patterns – we take after science. Each group experiences thorough testing in our cGMP-certified facilities.
We've created this detailing utilizing exclusive liposomal innovation that ensures NMN particles amid capacity and transport. This consideration to detail guarantees you get most extreme strength with each dose.
Our fabricating forms meet worldwide guidelines over over 50 nations. This worldwide involvement implies you're getting a item that meets the most noteworthy quality necessities worldwide.
Simple Integration into Your Routine
Our product offers adaptability for different applications. Blend it into refreshments, join it into capsules, or utilize it in custom definitions. The steady powder shape keeps up power without requiring extraordinary capacity conditions.
The clean name definition contains no superfluous added substances or allergens. This virtue makes it appropriate for different dietary inclinations and limitations.
Your Partner in Healthy Aging
Cellular health is the foundation of overall wellness. As we age, NAD+ levels naturally decline, affecting energy production and cellular repair processes. Supplementing with high-quality NMN helps maintain these crucial biological functions.
Our advanced delivery system ensures you're not just taking NMN – you're absorbing it effectively. This efficiency means you can use smaller doses while achieving better results compared to traditional formulations.
Get Started Today
Ready to experience the benefits of superior cellular energy support? Our product represents the next generation of anti-aging supplements, combining proven science with innovative delivery technology.
Contact us
For liposomal NMN powder information, custom formulations, or bulk orders, contact our team at info@emerwell-bio.com. We're here to support your health and wellness goals with science-backed solutions you can trust.
Address: 12549 Brickellia St, San Diego, CA 92129
Telephone: +1 (619) 736-0851
Take the first step toward optimized cellular health. Your future self will thank you for making this investment in longevity and vitality today.
For partners seeking the direct coenzyme form, we also offer premium Liposomal NAD+ Powder.
Have a project in mind? Tell us your goals — we’ll help you make it real.